Chemicals
Chemistry is a vast and diversified industry producing numerous products. It can be categorized in various ways:
- Based on the intended usage of its production:
- Commodity chemicals
 : or bulk chemicals are a group of chemicals that are made on a very large scale to satisfy global markets. Those are often intended to be refined further in other branches of the chemical industry.
: or bulk chemicals are a group of chemicals that are made on a very large scale to satisfy global markets. Those are often intended to be refined further in other branches of the chemical industry. - Speciality chemicals
 : are particular chemical products which provide a wide variety of effects on which many other industry sectors rely, such as adhesives, agrichemicals, cleaning materials, food additives, fragrances, industrial gases, lubricants, paints, polymers, etc. Speciality chemicals are materials used on the basis of their performance or function. Consequently, in addition to "effect" chemicals they are sometimes referred to as "performance" chemicals or "formulation" chemicals.
: are particular chemical products which provide a wide variety of effects on which many other industry sectors rely, such as adhesives, agrichemicals, cleaning materials, food additives, fragrances, industrial gases, lubricants, paints, polymers, etc. Speciality chemicals are materials used on the basis of their performance or function. Consequently, in addition to "effect" chemicals they are sometimes referred to as "performance" chemicals or "formulation" chemicals. - Fine chemicals
 : are complex, single, pure chemical substances, produced in limited quantities. They are described by exacting specifications, used for further processing within the chemical industry and this exclusivity makes them expensive.
: are complex, single, pure chemical substances, produced in limited quantities. They are described by exacting specifications, used for further processing within the chemical industry and this exclusivity makes them expensive. - Pharmaceuticals
 : are at the cross-road of chemistry and life sciences. It produces the drugs needed to diagnose, cure, treat, or prevent diseases.
: are at the cross-road of chemistry and life sciences. It produces the drugs needed to diagnose, cure, treat, or prevent diseases.
- Commodity chemicals
- Based on the nature of the constituents, even if the distinction between the two disciplines is far from absolute:
- Based on the where the products are sourced from:
We will focus mainly on commodity chemicals. An important group are petrochemicals, organic by nature. Another are fertilizers, inorganic by nature. And there are many other products, organic and inorganic, which are feedstock to the chemical industry itself or other industries, which are traded as commodities.
Products
Inorganic chemicals
Inorganic chemicals cover a very large range of products, some of which being used as a basic raw material in a variety of industries: chemical products, fibers, pulp and paper, water supply and sewage, fertilizers, food additives, pharmaceutical drugs, etc.
Organic chemicals
Petrochemicals are the chemical products obtained from hydrocarbons (gas, coal, petroleum) by refining. The two most common petrochemical classes are alkenes (also called olefins) and aromatics (BTX : benzene, toluene, xylene). Those are the building-blocks for a wide range of materials such as solvents, detergents, adhesives and muliple polymers used to produce plastics, resins, fibers, elastomers, lubricants, and gels.
| Group | Carbon atoms | ||||
|---|---|---|---|---|---|
| Label (IUPAC) | Structure | 1 | 2 | 3 | 4 |
| Alkanes |
Combinations of hydrogen and carbon atoms, whereby all the carbon–carbon bonds are single (general formula: CnH2n+2). | Methane (CH4) | Ethane (C2H6) | Propane (C3H8) | Butane (C4H10) |
| Alkyl |
An alkyl substituent is an alkane missing one hydrogen. | Methyl | Ethyl (C2H4) | Propyl (C3H6) | Butyl (C4H8) |
| Olefins (Alkene) |
A hydrocarbon that contains a carbon–carbon double bond. | Methylene | Ethylene, Ethene (C2H4) | Propylene, Propene (C3H6) | Butene (C4H8) |
| Vinyl (Ethenyl) |
Is the functional group with the formula −CH=CH2. | Ethenyl | Butadiene (C4H6) | ||
| Alcohol |
Organic compound that carries at least one hydroxyl functional group (−OH) bound to a saturated carbon atom. General formula is CnH2n+1OH | Methanol (CH3OH) | Ethanol (C2H5OH) | Propanol (C3H7OH) | Butanol (C4H9OH) |
| Diol |
A diol is a chemical compound containing two hydroxyl groups (−OH groups). | Ethyl glycol (C2H6O2) | |||
| Aromatics (Arene) |
Chemical compounds structured in rings. | Benzene (C6H6). The simpliest form of hydrocarbon aromatic: a hexagon of 6 carbon atoms. | |||
| Aryl |
Aryl is any functional group or substituent derived from an aromatic ring. Aryl with a functional group linked to a benzene ring is a phenyl group. |
Toluene, Methylbenzene (C6H5CH3) Xylene, Dimethylbenzene (C6H4(CH3)2 |
Ethylbenzene (C6H5CH2CH3) Styrene, Ethenylbenzene (C6H5CH=CH2) |
||
| Ether |
A class of organic compounds that contain an ether group: an oxygen atom connected to two alkyl or aryl groups. | Methyl tert-butyl ether | Ethyl tert-butyl ether | ||
| Polymers |
Material consisting of very large molecules (macromolecules), composed of many repeating subunits of chemical compounds. | Polyethylene Polystyrene |
Polypropylene | Polyvinylchloride | |
Feedstock to the entire petrochemicals chain ![]() are mainly produced from:
are mainly produced from:
- Natural gas, by steam cracking of gas liquids (alkanes), a process in which saturated hydrocarbons are broken down into smaller, often unsaturated, hydrocarbons.
- Petroleum, by steam cracking to produce olefins, or catalytic reforming of naphta to produce aromatics.
Due to the dependency on those feedstocks, petrochemicals are predominantly made in a few manufacturing locations around the world, close to oil refineries. Groups of related materials are often made in adjacent manufacturing plants to induce industrial symbiosis as well as material and utility efficiency and other economies of scale. In chemical engineering terminology: integrated manufacturing.
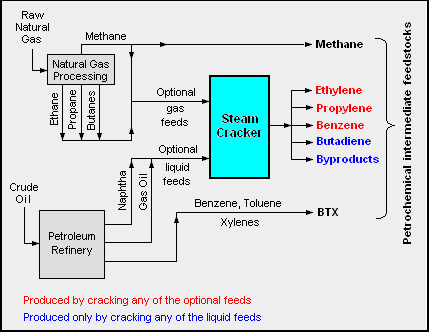
© Wikimedia
Fertilizers
A thorough insight into this industry is given by the International Fertilizer Industry Association (IFA) where we would recommend more particularly the excellent publication by Dr. Harold F. REETZ jr. (ISBN 979-10-92366-04-4) their website references.
Fertilizers are responsible for approximately half of the world’s crop production, supplying food, feed, fiber, and fuel for a growing global population. The extent to which world food production depends on fertilizer use will inevitably increase in future. It is projected that the world population will reach at least 9 billion people by 2050. As per FAO’s projection on world agriculture, global agricultural production in 2050 should be 60% higher than that of 2005/2007. An improving standard of living in much of the world will further add to the demand for food and fiber. At the same time there is an ongoing reduction in productive arable land so that mineral fertilizers will play a critical role in the world’s food security and will be important from both the yield and food quality perspectives. Without fertilizers, the world would produce only about half as much staple food, and more forested lands would have to be put into production.
There are 17 essential nutrients for crop growth:
- Fundamentals: Carbon (C), Hydrogen (H), and Oxygen (O) are supplied from air and water.
- Macronutrients: are mostly supplied from the soil, but soil deficiencies and crop removal must be replaced with supplemental sources, mostly fertilizers.
- Nitrogen (N): as N2 gas forms 78% of the Earth’s atmosphere and is non-reactive. It must be converted to reactive chemical forms (ammonium NO3 and nitrate NH4) to be utilized by plants. This conversion is done by micro-organisms in the soil, by symbiotic bacteria living on plants, or by chemical reactions.
- Phosphorus (P): usually occurs in large quantities in the soil minerals and organic matter, and must be converted to inorganic phosphate ions (H2PO4-) to be used by plants.
- Potassium (K): exists in large quantities in the soil minerals and adsorbed in the ionic form K+ to soil particles and organic matter. Potassium does not form any chemical compounds in plants, but plays a major role in transport of water and other ions across cell membranes.
- Secondary macronutrients: Sulfur (S), Calcium (Ca), Magnesium (Mg), are no less essential, but are usually needed in smaller amounts as fertilizers.
- Micronutrients: Boron (B), Iron (Fe), Manganese (Mn), Zinc (Zn), Copper (Cu), Molybdenum (Mo), Chlorine (Cl), Nickel (Ni), are needed in very small amounts, but play essential roles as catalysts in metabolic processes of crop growth and development or play other key roles.
Most fertilizer materials come from concentrated supplies of naturally-occurring minerals that are mined or extracted from various ore deposits. One exception is nitrogen (N) which is produced by combining N2 from the air with natural gas (most common), coal, or naphtha to form anhydrous ammonia, which can be used directly as a fertilizer or converted to different other nitrogen fertilizers.
Beyond supplying minerals, fertilizers are often sold as manufactured products, blending different nutrients. They are graded on basis of the available major 3 plant nutrients expressed as a percentage by weight: Nitrogen (N) expressed as total content, Phosphorus (P) and Potassium (K) expressed as oxide form. Sulfur content is sometimes added as a fourth number.
| NPK : 7-28-14 | ||
| Nitrogen | Phosphorus | Potassium |
| 7% N |
28% P2O5 ≅ 64% P [P2O5 = 2.29 P] |
14% K2O ≅ 17% K [K2O = 1.20 K] |
Blends consider soil requirements, harvested crop, chemical and physical compatibility, nutrient release, intake rythm, and processing efficiency. Combination with other products, such as micro-nutrients or herbicides, exist as well. Fertilizers also come in different forms such as:
- Crystalline, Powder
- Granular: Prilled, Coated.
- Liquid: diluted or suspended, for focused ferilization or added to irrigation water.
- Gaseous: gas injected under pressure (such as ammonia).
Next to the uncontested positive contribution of fertilizers to crop production, some negative impacts need to be mentioned:
- Nitrogen (as nitrate NO3-) and Phosphorus may be lost in soil water through leaching, runoff from the soil surface or soil erosion. In surface water bodies, they support growth of algae and aquatic plants, which as they die and decompose, tie up oxygen in the water, creating a hypoxic condition which starves aquatic animals for oxygen.
- Nitrogen may be lost into the atmosphere from the soil or from growing plants as inert N2 gas, ammonia (NH3), nitrous oxide (N2O) or NOx gases. The latter two are over 300 times as potent as CO2 as a greenhouse gas, contributing to global warming.
